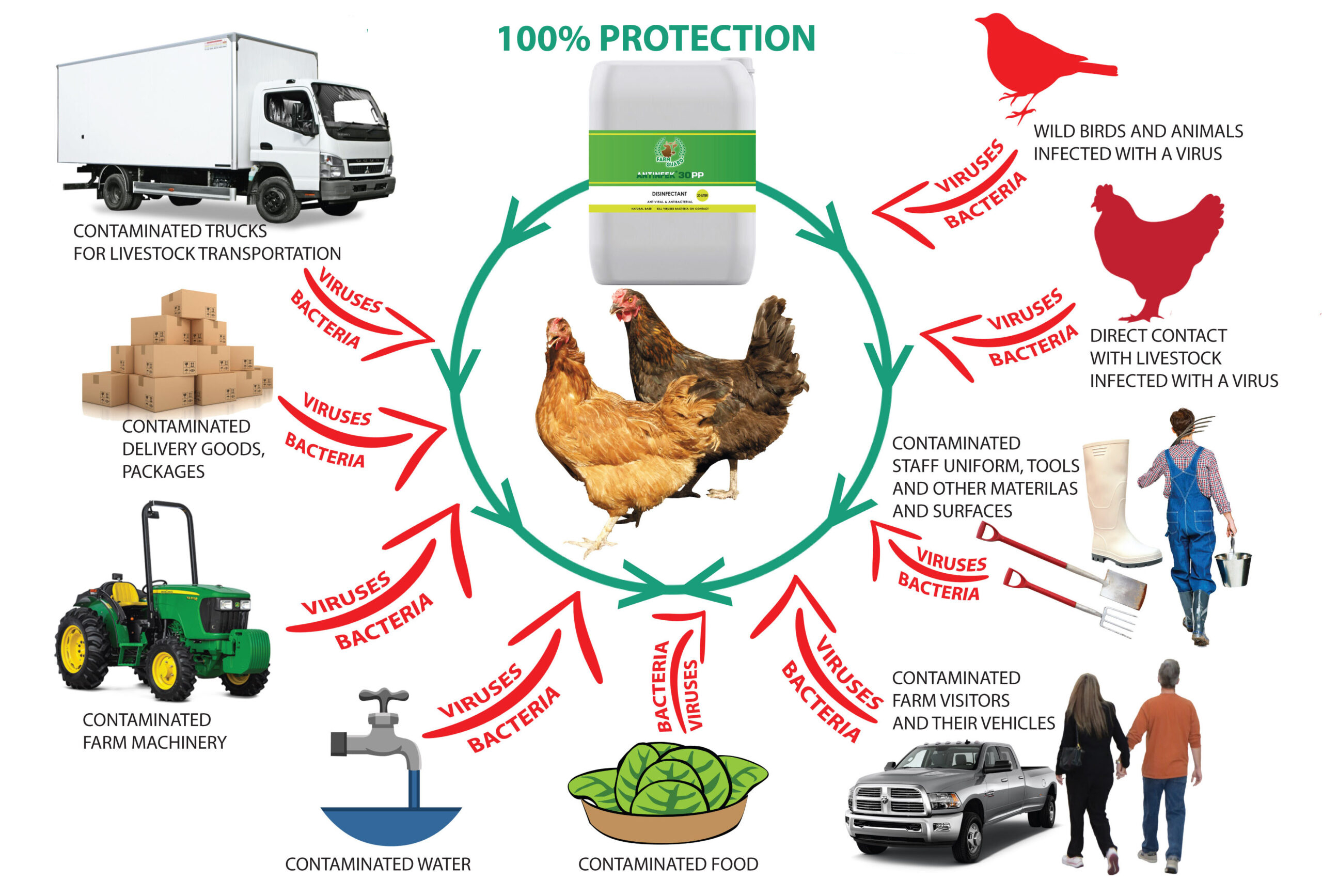
The Common Disinfectants for poultry disinfection
Common Disinfectants
The ideal disinfectant is one that acts quickly, kills pathogens at low concentrations, has high bactericidal properties; does not have a destructive effect on metals, wood, and plastic products; is not easy to explode, and is safe to use; is non-toxic and has no side effects for humans and chickens; is relatively stable in price, has no odor, and is soluble in water; and will not be affected by the presence of external protein, exudate, or organic matter. However, under current conditions, some disinfectants have some drawbacks to a greater or lesser extent. When using them, they should be selected according to the object to be disinfected and the purpose of disinfection.
1. Phenols
(1)Common drugs
The earliest phenols were phenol (carbolic acid), which was corrosive and irritating to the tissues and is now rarely used. Common phenol preparations include chloramine (stinking water), compound phenol preparations, and creolin (lysol).
(2)Dosage
Chloramine and creolin solution are commonly used at a concentration of 3%~5% for disinfection of chicken houses and utensils, and the concentration for disinfection of excrement is 5%~10%; they can also be used at a concentration of 100% for bathing, treating chicken lime foot disease. The imported disinfectant compound phenol Nongfu is sprayed at a concentration of 0.15%~1%.
(3)Precautions
Compound phenol is a broad-spectrum and highly effective disinfectant that can be used to kill bacteria, fungi, and viruses. Compound phenol is a dark, viscous liquid with a peculiar odor and usually remains effective for 7 days. Do not mix other disinfectants or alkaline drugs when spraying compound phenol.
2. Acids
(1)Common drugs
Common organic acid disinfectants are lactic acid and acetic acid, which are often used for air disinfection.
(2)Sterilization principle
The production process uses organic acids rather than inorganic acids, because the sterilization principle of organic acids is to use ionized molecules to kill bacteria through the lipid bilayer of the bacterial cytoplasm, while inorganic acids use hydrogen ions to kill bacteria.
(3)Dosage
Use 10 ml of lactic acid and 10~20 ml of water for each cubic meter of space, and heat to vaporize; or use 20~40 ml of dilute acetic acid to heat to vaporize. If it is vinegar for eating, each cubic meter needs 300~1000 ml.
3. Alkalis
(1)Common drugs
The main alkaline disinfectants are sodium hydroxide (caustic soda), potassium hydroxide (caustic potash), and lime.
(2)Sterilization principle
The sterilization effect of alkaline disinfectants is related to the concentration of hydroxide ions. The higher the concentration, the stronger the bactericidal ability. Under room temperature conditions, strong alkali can hydrolyze proteins and nucleic acids, damage the enzymes and cell structure of bacteria, and disrupt the metabolic process of bacteria, leading to bacterial death. Alkaline disinfectants are especially effective against gram-negative bacteria, and they are also effective in killing viruses. High-concentration alkaline liquids also work on spores.
(3)Dosage
2% sodium hydroxide or potassium hydroxide is used for disinfection of virus and bacterial contamination. The commonly used industrial flake alkali contains 94% sodium hydroxide. Lime, when hydrolyzed, releases hydroxide ions, which acts as a disinfectant. Lime water must be made up immediately, and the commonly used concentration is 10%~20%.
(4)Precautions
Lime is effective against general bacteria, but it is ineffective against spores and tubercle bacilli. It is commonly used for disinfection of floors, walls, and manure pits.

4. Oxidizing Agents
(1) Common drugs
Common oxidizing disinfectants are potassium permanganate (manganese oxide) and hydrogen peroxide (acetic acid).
(2) Disinfection principle
Oxidizing disinfectants contain unstable bound oxygen, which releases primary oxygen when it comes into contact with organic matter or enzymes, destroying bacterial proteins and enzyme proteins to exert a bactericidal effect, especially effective against anaerobic bacteria, and effective against Gram-positive bacteria.
(3) Dosage
A 0.1% potassium permanganate solution is used for skin, mucous membranes, wound washing and drinking water disinfection; a concentration of 2.5% can kill spores. Hydrogen peroxide has a pungent odor and is explosive at high concentrations. The general market product has a concentration of 20%, with no explosion hazard and a shelf life of half a year. Hydrogen peroxide has strong oxidation properties, strong bactericidal effect, broad spectrum of activity, effective against bacteria, viruses, fungi and spores. A 0.2%~0.5% hydrogen peroxide solution is used for disinfection of chicken coop environment, and it should be used as soon as it is prepared. In the fumigation disinfection of formaldehyde solution, potassium permanganate is often used, but at this time it is used to accelerate the release of formaldehyde solution by taking advantage of its oxidation properties.
(4) Precautions
Hydrogen peroxide is irritating and corrosive to tissues, and it is also corrosive to metals. Safety precautions must be taken when using it.
5. Halogens
(1) Common drugs
Halogen disinfectants mainly include chlorine, bromine, iodine preparations, bleaching powder (containing chlorine lime), and sodium dichloroisocyanurate (Super Chlorine). The most common chlorine preparations.
(2) Disinfection principle
Because chlorine is very easy to penetrate into the cells of bacteria, it produces halogenation and oxidation reactions with proteins, with strong bactericidal activity; but chlorine is a gas, which is inconvenient to use. In general, compounds that release free chlorine are used as disinfectants. These products have a variety of brand names, but the sterilization principle is similar.
(3) Dosage
Bleach is a mixture of calcium hypochlorite, calcium chloride and calcium hydroxide. It should contain 25%~30% of available chlorine, and products with available chlorine below 16% should not be used; it is mainly used for drinking water disinfection, and generally 6 mg/L of available chlorine should be maintained in the water. Sodium dichloroisocyanurate contains more than 60% of available chlorine, is stable in nature, and its 0.5%~1% concentration is used to kill fire bacteria and viruses, and 5%~10% is used to kill bacterial spores. When drinking water is disinfected, 4 mg is used per liter of water, and it can be drunk after 30 minutes. Iodine has a strong bactericidal effect against bacteria, viruses and fungi, and its disinfection object is almost non-selective, with the same effective concentration for various microorganisms; in drinking water disinfection, 100~150 ml of 2% iodine tincture is added to one ton of water, and it can be drunk after 15 minutes. It is used at a concentration of 2%~5% for skin disinfection.
6. Anionic Surfactants
(1) Common drugs
Anionic surfactants such as chlorhexidine, chlorhexidine gluconate, and chlorhexidine digluconate.
(2) Disinfection principle
Surfactants have strong antibacterial effects, which are commonly used disinfectants in clinical practice. Its antibacterial principle is that the hydrophilic and lipophilic groups of the active agent penetrate into the lipid bilayer and protein layer of the cytoplasm, thereby changing the permeability of the cytoplasm membrane and causing the release of intracellular substances to exert a bactericidal effect.
(3) Precautions
Anionic surfactants are most effective in alkaline environments, and the bactericidal effect is significantly reduced in acidic environments. Anionic surfactants have a wide range of bactericidal effects against various bacteria, viruses and fungi. At the same time, it has the advantages of strong bactericidal effect, rapid action, low irritation, low toxicity, small dosage, long-term storage and low price, which is a relatively ideal disinfectant.
(4) Dosage
① Iodine is a complex of iodine and surfactants, which can be used for washing and soaking disinfection, generally diluted 10~20 times after use; it can also be used for drinking water disinfection, adding 10~20 ml of the original drug liquid per liter of water, and drinking for 3~5 days; it can also be used for disinfection of chickens

7. Volatile Alkylating Agents
(1) Common drugs
The most common volatile alkylating agents are formaldehyde solution and ethylene oxide.
(2) Disinfection principle
Volatile alkylating agents are very active at room temperature, and can react with unstable hydrogen atoms such as bacterial proteins, nucleic acids, and hydroxyl groups to undergo alkylation reactions, which can change the function of bacterial proteins or nucleic acids and exert a bactericidal effect. This type of preparation is effective against bacteria, spores, viruses, fungi, and even insects and insect eggs. It is also a gaseous disinfectant, which is especially suitable for use in cases where liquid disinfection is not suitable.
(3) Dosage
Formaldehyde solution and ethylene oxide are used for fumigation disinfection, with a dosage of 15~30 ml per cubic meter of space. The solution can be heated to evaporate after adding an equal amount of water, or it can be oxidized and evaporated by adding half the amount of potassium permanganate. The temperature of the chicken coop should be at least 20℃ and the relative humidity should be at least 60% during fumigation disinfection. For fumigation disinfection of newly hatched chicks, use 7 ml of formaldehyde solution, 3.5 ml of water, and 3.5 grams of potassium permanganate per cubic meter of space, and fumigate for 30~45 minutes.








